Data Blitz A: Skin burns
Epidermal stem cells are among the few stem cell types that are already used in the treatment of patients. Thanks to a discovery made in the USA in 1970 by Professor Howard Green, epidermal stem cells can be taken from a patient, duplicated and used to grow new epidermis in the laboratory. This epidermis can then be used as a skin graft for the patient. In the interim, researchers in a laboratory have succeeded in growing up to 20 skin grafts of 60 square centimeters each in just three weeks from the cells of a small piece of skin. As the new skin was grown from the body's own cells, it is not rejected. The technique is mainly used to save the lives of patients who have suffered large-scale third-degree burns. Only a handful of clinical centers are capable of successfully performing this costly treatment. However, it is not an ideal solution. Using this method, only the epidermis can be replaced; the
new skin has no skin appendages, such as hair follicles, sweat glands or sebaceous glands. In particular, the absence of the sweat glands affects the patients, as they do not have normal thermoregulation. Intensive research is being performed to improve the properties of the grown skin.is mainly used to save the lives of patients who have suffered large-scale third-degree burns. Only a handful of clinical centers are capable of successfully performing this costly treatment. However, it is not an ideal solution. Using this method, only the epidermis can be replaced; the new skin has no skin appendages, such as hair follicles, sweat glands or sebaceous glands. In particular, the absence of the sweat glands affects the patients, as they do not have normal thermoregulation. Intensive research is being performed to improve the properties of the grown skin.
Read more: Skin stem cells: Where do they live and what can they do
Data Blitz B: Liver failure
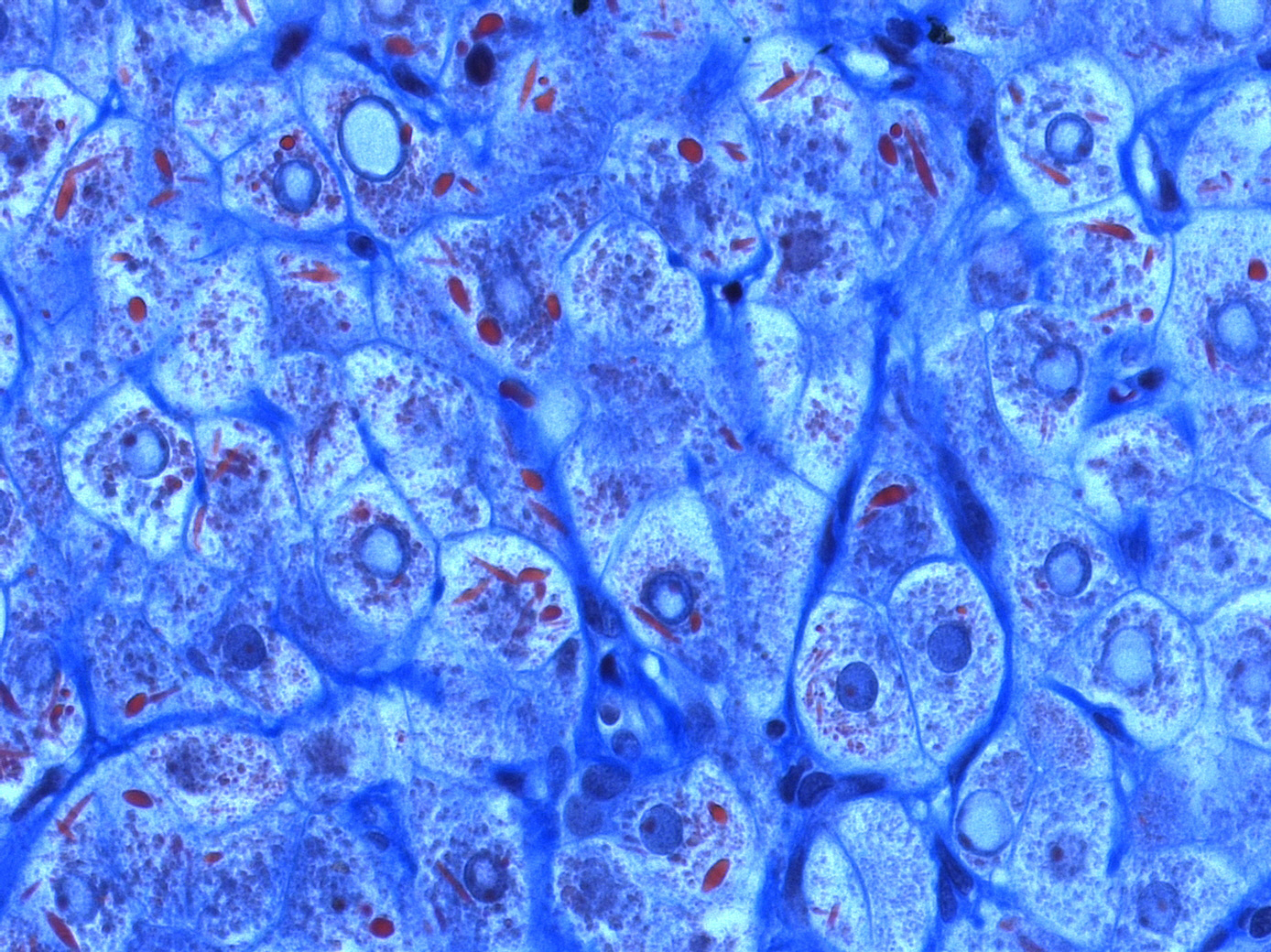 Foto: Mega-mitochondria of hepatocytes under light microscope (using chromotrope anillin blue stain, CAB)
Foto: Mega-mitochondria of hepatocytes under light microscope (using chromotrope anillin blue stain, CAB)
Source: Alexquaas/Wikimedia
For a long time, a liver transplant was the only treatment option for acute liver failure. This will hopefully change soon – thanks to stem cell therapy. Liver cells (hepatocytes) from the laboratory can be used as an interim solution. In order to transfer them to the liver cell culture, natural liver formation is being researched. Growth agents (cytokines) are added and reagents are developed that will fine-tune cell characteristics of gene expression. MicroRNAs, that is, non-coding RNA molecules, can also play an important role in this process. The advantage of this procedure: The patient's own cells are used, meaning that no immunosuppressant drugs are necessary. Research is also being carried out to convert the body's own connective tissue cells in the liver into hepatocytes, allowing them to participate in the healing process.

Find out more: GSCN Video - The liver: Therapy with stem cells -
The research of Prof. Michael Manns and Prof. Tobias Cantz (MH Hannover)
Download movie offline MP4
Read more: Chronic liver disease: How could regenerative medicine help?
Data Blitz C: Corneal clouding
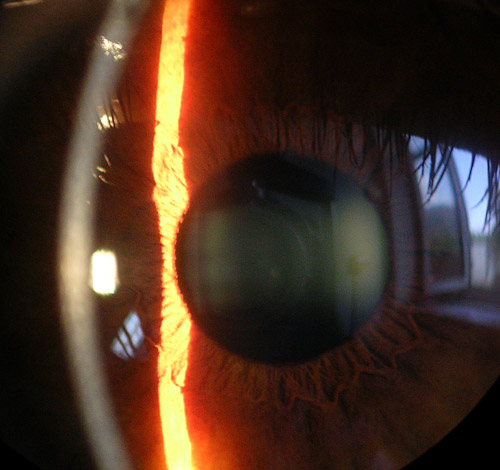 Foto: Picture of the cornea, iris and lens with a slit lamp
Foto: Picture of the cornea, iris and lens with a slit lamp
Source: Baristoprak~commonswiki/Wikimedia
James Funderburgh of the University of Pittsburgh, together with ophthalmologists from India, investigated whether stem cells could be used to heal corneal injuries. For their experiments, they originally used the limbus stem cells of deceased people. The limbus is the transition zone between the cornea and the dermis in the eyeball. Today, for clinical applications, the cells are taken from the patient's healthy eye limbus. According to the researchers, such a biopsy can be kept so small that it does not endanger the healthy eye. The cells are then first multiplied in the laboratory, which is easily possible today. The stem cells are then applied directly to the injured cornea using a fibrin glue. Transparency and vision returns within four weeks. In the meantime, the product Holoclar, which was approved for patient treatment on the European market, has become available for these treatment options.
Read more:
- The eye and stem cells: the path to treating blindness
- Europe approves Holoclar first stem cell based medicinal product
Data Blitz D: Leukemia
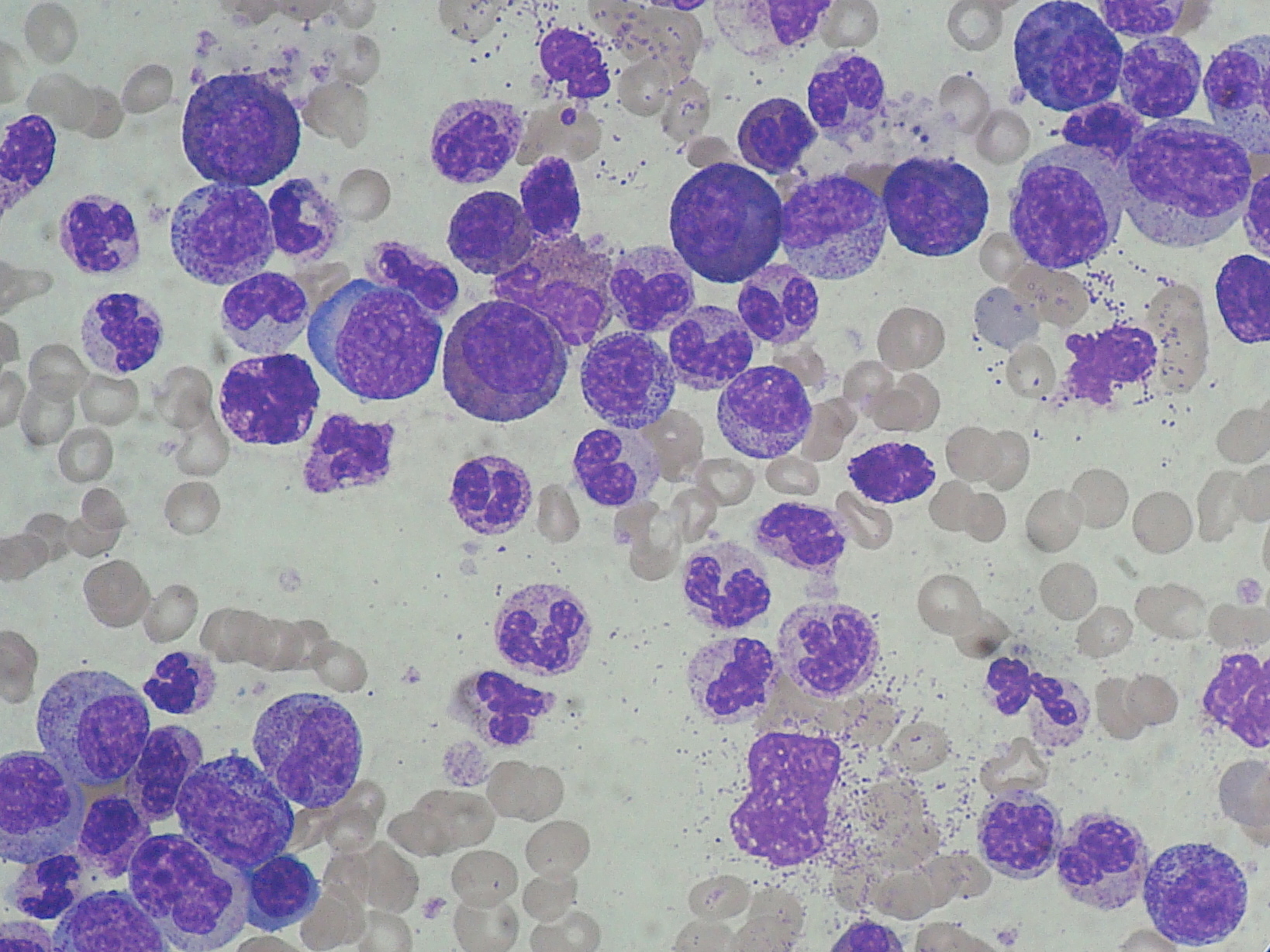 Foto: Chronic myeloid leukemia (CML): marked leucocytosis with granulocyte left shift.
Foto: Chronic myeloid leukemia (CML): marked leucocytosis with granulocyte left shift.
Source: Paulo Henrique Orlandi Mourao/Wikimedia
In leukemia, blood stem cells have lost their ability to differentiate correctly. The cells renew themselves without becoming specialists. The leukemia cells produce white precursor cells that do not mature and are not functional. They spread within the bone marrow and interfere with normal blood formation. As a consequence, the blood no longer carries enough oxygen and loses its wound healing function. Blood stem cells are transplanted to treat leukemia. In the process, the autologous blood stem cells causing the leukemia are destroyed with all hematopoietic cells through high doses of chemotherapy or radiation. In a second step, the blood stem cells previously obtained from the patient are returned (autologous) or transplanted from a suitable donor (allogeneic). Allogeneic stem cell transplantation is necessary if the leukemia has developed in the patient's own blood stem cells. However, it carries the risk of graft-versus-host disease (GvHD), in which the immune cells of the donor attack the patient's organs.

Find out more: GSCN Video about the cell therapie with CRISPR/Cas -
The research of Hubert Serve (University hospital Frankfurt)
Download movie offline MP4
Read more: Leukemia: How can stem cells help?
Data Blitz E: Diabetes
Douglas Melton's children have diabetes and need to inject insulin. The internationally-renowned Harvard professor intends to change this.
From the Neue Zürcher Zeitung, Oct. 7, 2016:
“In type 1 diabetes, only one type of cell is missing, which in addition performs an absolutely crucial task in its organ, the pancreas: measure the blood sugar level and, if necessary, dispense insulin. This must be cured by replacement cells grown in the laboratory,” says Melton, was convinced even back then. So, he began growing these replacement cells. (...) It would take until 2014 for the Harvard team to make functioning beta cells from embryonic stem cells as well as from iPS cells – the hoped for breakthrough. The grown beta cells measure glucose concentrations, produce insulin and emit it when needed. This works both in the artificial environment of a Petri dish and in diabetic mice. Elsewhere in the world, other researchers were also working toward this goal, but no one was faster than Melton. Some also try to reprogram cells into beta cells directly within the pancreas.
“There are at least 70 proteins in the body that control the development and specialization of body cells from embryonic stem cells. We had to find those that create beta cells, in the correct temporal combination and concentration,” says Melton, describing the greatest hurdle.
Other questions also remain unanswered. It is still unclear whether iPS or embryonic stem cells are the better source material. For standardized therapies, the latter will probably prevail, because once here, a stem cell line and the beta cells grown from it can be subjected to all safety checks and then be used in every patient. When using iPS cells derived from the patient to be treated, the safety tests would need to be performed individually for each patient – too costly and expensive for clinical use.
In addition, we do not yet know how many newly grown beta cells we need to control blood sugar levels and how long they will work in the body. Answers to such questions could be provided by the world's first attempt to treat diabetes using replacement cells, started in late-2014 by the California company ViaCyte. In the process, beta-cell precursors grown from stem cells and packaged in biopolymer capsules were implanted. According to the company, initial results reveal that at least some of the implanted cells are mature after 12 weeks. How well they work and how long they will survive is still uncertain.”
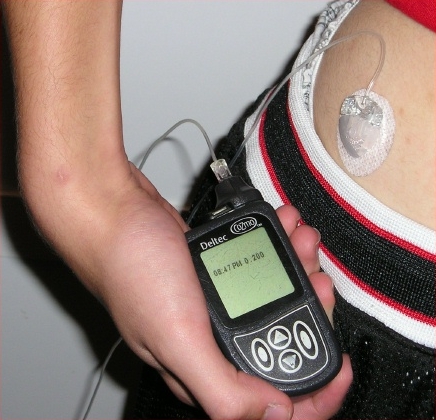
Foto: Insulin pump with infusion set
Source: mbbradford/Wikimedia
Read more: Diabetes: how could stem cells help?
Data Blitz F: Parkinson's disease
Physicians and scientists are of the opinion that cell replacement therapy can also work for the neurodegenerative Parkinson's disease, citing the results of transplantation studies conducted in the 1980s. Scandinavian scientists had transplanted adrenal cells into the brains of four Parkinson's disease patients. The adrenal glands are located on the kidneys and contain cells that produce dopamine and similar substances. Following transplantation, some improvement occurred in the condition of the patients, but was, however, slight and did not last long. This was the first transplantation of dopamine-producing tissue into the human brain. In subsequent tests, scientists from Sweden, the USA and Canada transplanted dopamine-producing neurons from human fetuses into animals and patients and thereby achieved some crucial, but only modest, improvements. One group of patients suffered side effects and, in some cases, more than a decade after the procedure, the disease was also transferred to the transplanted fetal cells.
.jpg)
Illustration: Photomicrograph of regions of substantia nigra in a Parkinson's patient showing Lewy bodies and Lewy neurites in various magnifications. Top panels show a 60-times magnification of the alpha synuclein intraneuronal inclusions aggregated to form Lewy bodies. The bottom panels are 20 × magnification images that show strand-like Lewy neurites and rounded Lewy bodies of various sizes. Neuromelanin laden cells of the substantia nigra are visible in the background.
Source: Suraj Rajan/Wikimedia
Scientists still hope to delay the onset or slow down the advance of Parkinson's disease by introducing cells from early stages of human development into the brain. However, there is insufficient fetal tissue available to treat the large number of Parkinson's disease patients. In addition, the use of fetuses poses ethical questions. Stem cells could serve as an alternative source of new cells for Parkinson's disease patients. Embryonic stem cells (ES) could be made to produce dopamine-producing neurons that would be transplanted into the patient. In the laboratory, dopamine-producing neurons have been produced both from embryonic stem cells from mice and from humans. Induced pluripotent stem cells (iPS) could be prepared in the laboratory from differentiated skin cells of patients and then be used to form dopamine-producing neurons. In 2010, American scientists treated rats with neurons produced from human skin cells using the iPS procedure. The transplanted neurons caused a reduction in Parkinson's disease symptoms in the rats. However, mice and rats require fewer neurons than humans, and it remains questionable whether this approach will work in humans – clinical trials to test this are in preparation worldwide. Furthermore, additional studies are required to demonstrate that treatment using the cells is safe and does not cause brain tumors.
Read more: Parkinson's disease: How could stem cells help?